Does Silver Nitrate Conduct Electricity . when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: If we want to electroplate a steel fork with silver: metals conduct electricity because they have delocalised electrons. These carry electrical charge through the metal. the object to be electroplated must be made of an electrical conductor. To determine of the solution is a strong or weak electrolyte; without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. 2 agno 3 2 ag + o 2 + 2 no 2. 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. The anode will be a. to observe electrical conductivity of substances in various aqueous solutions;
from www.alamy.com
To determine of the solution is a strong or weak electrolyte; the object to be electroplated must be made of an electrical conductor. 2 agno 3 2 ag + o 2 + 2 no 2. If we want to electroplate a steel fork with silver: without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. The anode will be a. metals conduct electricity because they have delocalised electrons. These carry electrical charge through the metal. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide:
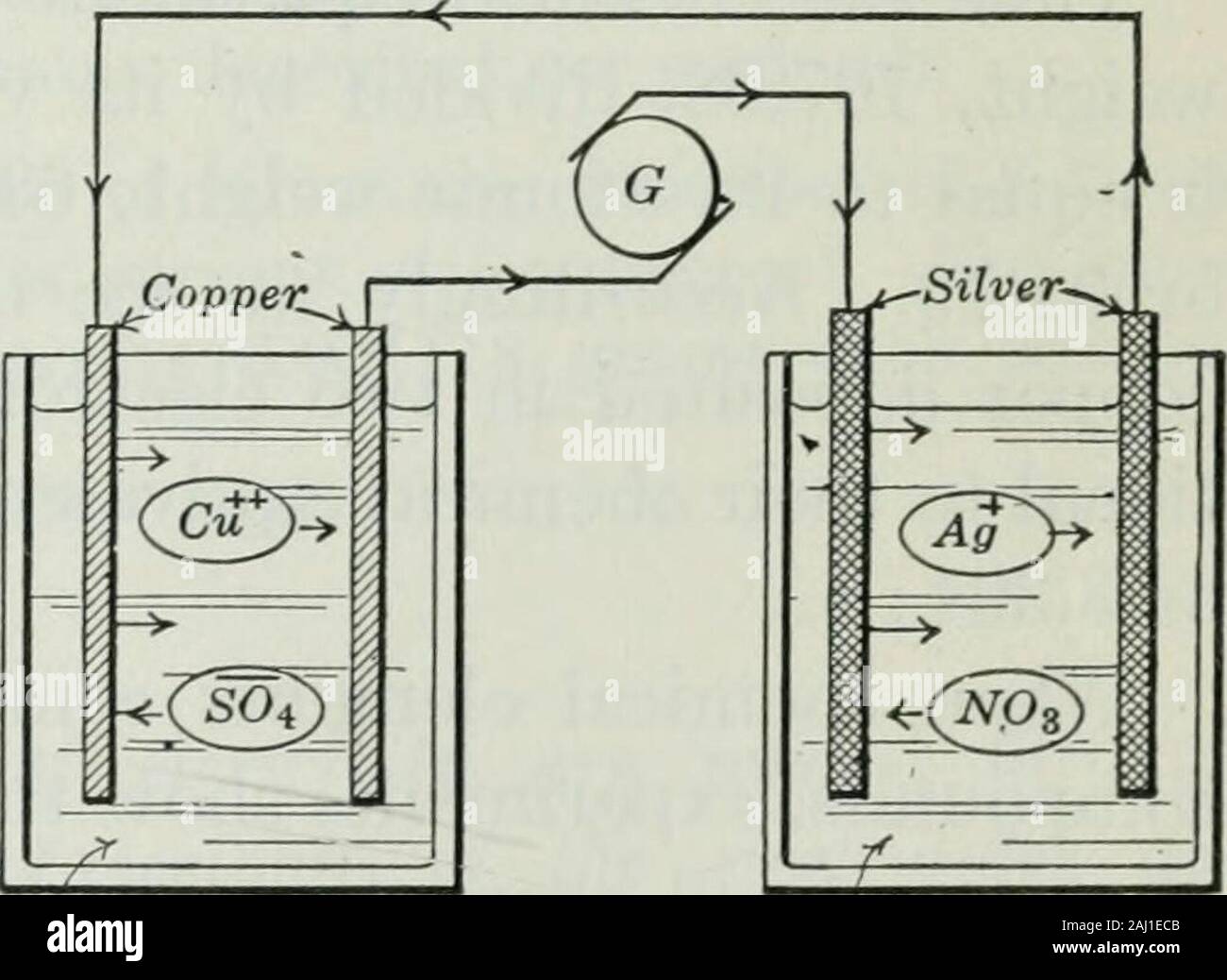
An elementary book on electricity and and their applications
Does Silver Nitrate Conduct Electricity to observe electrical conductivity of substances in various aqueous solutions; metals conduct electricity because they have delocalised electrons. 2 agno 3 2 ag + o 2 + 2 no 2. If we want to electroplate a steel fork with silver: The anode will be a. the object to be electroplated must be made of an electrical conductor. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: To determine of the solution is a strong or weak electrolyte; without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. to observe electrical conductivity of substances in various aqueous solutions; 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. These carry electrical charge through the metal.
From data.epo.org
Silver nitrate produced by a continuous evaporative crystallization Does Silver Nitrate Conduct Electricity If we want to electroplate a steel fork with silver: 2 agno 3 2 ag + o 2 + 2 no 2. the object to be electroplated must be made of an electrical conductor. These carry electrical charge through the metal. to observe electrical conductivity of substances in various aqueous solutions; 37 rows by the same token,. Does Silver Nitrate Conduct Electricity.
From askfilo.com
What is the formula for silver nitrate? Filo Does Silver Nitrate Conduct Electricity These carry electrical charge through the metal. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. metals conduct electricity because they have delocalised electrons. The anode will be a. 2 agno 3 2 ag + o. Does Silver Nitrate Conduct Electricity.
From ar.inspiredpencil.com
Silver Nitrate Solution Color Does Silver Nitrate Conduct Electricity The anode will be a. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. If we want. Does Silver Nitrate Conduct Electricity.
From ar.inspiredpencil.com
Silver Nitrate Structure Does Silver Nitrate Conduct Electricity to observe electrical conductivity of substances in various aqueous solutions; metals conduct electricity because they have delocalised electrons. These carry electrical charge through the metal. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: To determine of the solution is a strong or weak electrolyte; 37 rows by the same token, the most. Does Silver Nitrate Conduct Electricity.
From www.youtube.com
How does Silver nitrate react with Potassium phosphate? K2HPO4 Does Silver Nitrate Conduct Electricity The anode will be a. These carry electrical charge through the metal. without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. to observe electrical conductivity of substances in various aqueous solutions; 2 agno 3 2 ag + o 2 + 2 no 2. If we want to electroplate a. Does Silver Nitrate Conduct Electricity.
From data.epo.org
Silver nitrate produced by a continuous evaporative crystallization Does Silver Nitrate Conduct Electricity without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. 2 agno 3 2 ag + o 2 + 2 no 2. 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. To determine of the solution is a strong or. Does Silver Nitrate Conduct Electricity.
From www.poolzoom.com
Reagent Silver Nitrate 3/4 oz. Does Silver Nitrate Conduct Electricity the object to be electroplated must be made of an electrical conductor. without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. These carry electrical charge through the metal. If we want to electroplate a steel fork with silver: The anode will be a. to observe electrical conductivity of. Does Silver Nitrate Conduct Electricity.
From slideplayer.com
Silver Nitrate Lab Day 2 Limiting Reactants ppt download Does Silver Nitrate Conduct Electricity the object to be electroplated must be made of an electrical conductor. If we want to electroplate a steel fork with silver: The anode will be a. 2 agno 3 2 ag + o 2 + 2 no 2. To determine of the solution is a strong or weak electrolyte; without a supporting electrolyte, adding the silver nitrate. Does Silver Nitrate Conduct Electricity.
From www.researchgate.net
Effect of silver nitrate concentration on AgNPs synthesis Download Does Silver Nitrate Conduct Electricity The anode will be a. To determine of the solution is a strong or weak electrolyte; the object to be electroplated must be made of an electrical conductor. 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. without a supporting electrolyte, adding the silver nitrate may have. Does Silver Nitrate Conduct Electricity.
From www.labvalley.com
Silver nitrate solution 0.1N (0.1 mol/L) (1 ลิตร/ขวด) ยี่ห้อ Fisher Does Silver Nitrate Conduct Electricity If we want to electroplate a steel fork with silver: These carry electrical charge through the metal. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. 2 agno 3 2 ag + o 2 + 2. Does Silver Nitrate Conduct Electricity.
From www.researchgate.net
The effect of nano silver nitrate zinc source in MS medium and their Does Silver Nitrate Conduct Electricity the object to be electroplated must be made of an electrical conductor. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. These carry electrical charge through the metal. metals conduct electricity because they have delocalised. Does Silver Nitrate Conduct Electricity.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Does Silver Nitrate Conduct Electricity These carry electrical charge through the metal. metals conduct electricity because they have delocalised electrons. The anode will be a. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: the object to be electroplated must be made of an electrical conductor. without a supporting electrolyte, adding the silver nitrate may have a large. Does Silver Nitrate Conduct Electricity.
From www.indiamart.com
Silver Nitrate Powder, 1Kg at Rs 48950/kg in Mumbai ID 11126193930 Does Silver Nitrate Conduct Electricity If we want to electroplate a steel fork with silver: the object to be electroplated must be made of an electrical conductor. to observe electrical conductivity of substances in various aqueous solutions; 2 agno 3 2 ag + o 2 + 2 no 2. without a supporting electrolyte, adding the silver nitrate may have a large effect. Does Silver Nitrate Conduct Electricity.
From www.alamy.com
An elementary book on electricity and and their applications Does Silver Nitrate Conduct Electricity If we want to electroplate a steel fork with silver: when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: These carry electrical charge through the metal. the object to be electroplated must be made of an electrical conductor. To determine of the solution is a strong or weak electrolyte; to observe electrical conductivity of. Does Silver Nitrate Conduct Electricity.
From www.researchgate.net
Optimization of AgNPs with different ratio of silver nitrate and ELE Does Silver Nitrate Conduct Electricity when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: to observe electrical conductivity of substances in various aqueous solutions; 2 agno 3 2 ag + o 2 + 2 no 2. The anode will be a. metals conduct electricity because they have delocalised electrons. the object to be electroplated must be made of. Does Silver Nitrate Conduct Electricity.
From www.youtube.com
The Reaction Between Silver Nitrate and Sodium Chloride YouTube Does Silver Nitrate Conduct Electricity to observe electrical conductivity of substances in various aqueous solutions; without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. To determine of the solution is a strong or weak electrolyte; The anode will be a. when heated, silver nitrate decomposes into metallic silver, oxygen and nitrogen oxide: . Does Silver Nitrate Conduct Electricity.
From lessonlibraryselfist.z21.web.core.windows.net
Salt Water And Electricity Does Silver Nitrate Conduct Electricity These carry electrical charge through the metal. without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. To determine of the solution is a strong or weak electrolyte; to observe. Does Silver Nitrate Conduct Electricity.
From www.researchgate.net
Vial containing the mixture of silver nitrate (100 mM) and flavonoids Does Silver Nitrate Conduct Electricity If we want to electroplate a steel fork with silver: without a supporting electrolyte, adding the silver nitrate may have a large effect on the potential of the. metals conduct electricity because they have delocalised electrons. 37 rows by the same token, the most effective conductors of electricity are metals that have a single valence. to. Does Silver Nitrate Conduct Electricity.